New studies highlight the use and limitations of teeth as ecological proxies in ancient sharks
In 2023, I published my first PhD chapter as a scientific paper – producing a framework to infer shark traits from tooth measurements. Being based on living sharks, I would subsequently apply this framework to Cenozoic sharks where many species have living representatives. Here, I explore how my work has been applied to sharks even earlier than this and the fascinating results those studies have found beyond even what I expected was possible.
The tooth, the whole tooth, and nothing but the tooth
When it comes to fossil sharks, teeth are typically all that remain for palaeontologists to study. With a soft cartilaginous skeleton that decomposes after a shark dies, there are two reasons why their teeth are such common fossils. Firstly, they are much harder than cartilage and therefore preserve better. Secondly, sharks are constantly losing teeth, going through thousands in their lifetimes. This perfect and unique combination of good preservation and constant loss and replacement has made shark teeth some of the most common remains in the marine vertebrate fossil record (Kent 1994; Hubbell 1996; Cappetta 2012).
While there is naturally some uncertainty and limitations in using fossil proxies to infer size or shape of extinct taxa (Gayford et al. 2024), teeth can sometimes be the only available tools to us to infer ecological traits like size or diet in fossil sharks. So exactly how well teeth can be used as proxies for these traits and others is a question that shark scientists have been asking for decades, with a series of mixed results over the years (Cooper et al. 2023 and references therein). This is partially because we are often forced to use living sharks as direct ecological analogues, which aren’t always a natural one-to-one fit with a fossil known only from similar teeth.
My PhD at Swansea University focused on shark functional diversity – the diversity of their ecological roles quantified by combinations of traits (Mouillot et al. 2013; Cooper 2024). Specifically, I wanted to focus on how shark functional diversity changed through time, including through the fossil record (Cooper 2024; Cooper & Pimiento 2024). The main issue with this, of course, was the inability to measure traits such as body size, prey preference and feeding mechanism directly in extinct sharks. So, for the first paper of my PhD, the goal was to understand if isolated teeth common to the shark fossil record could be accurate proxies for these traits in living sharks (Cooper et al. 2023).
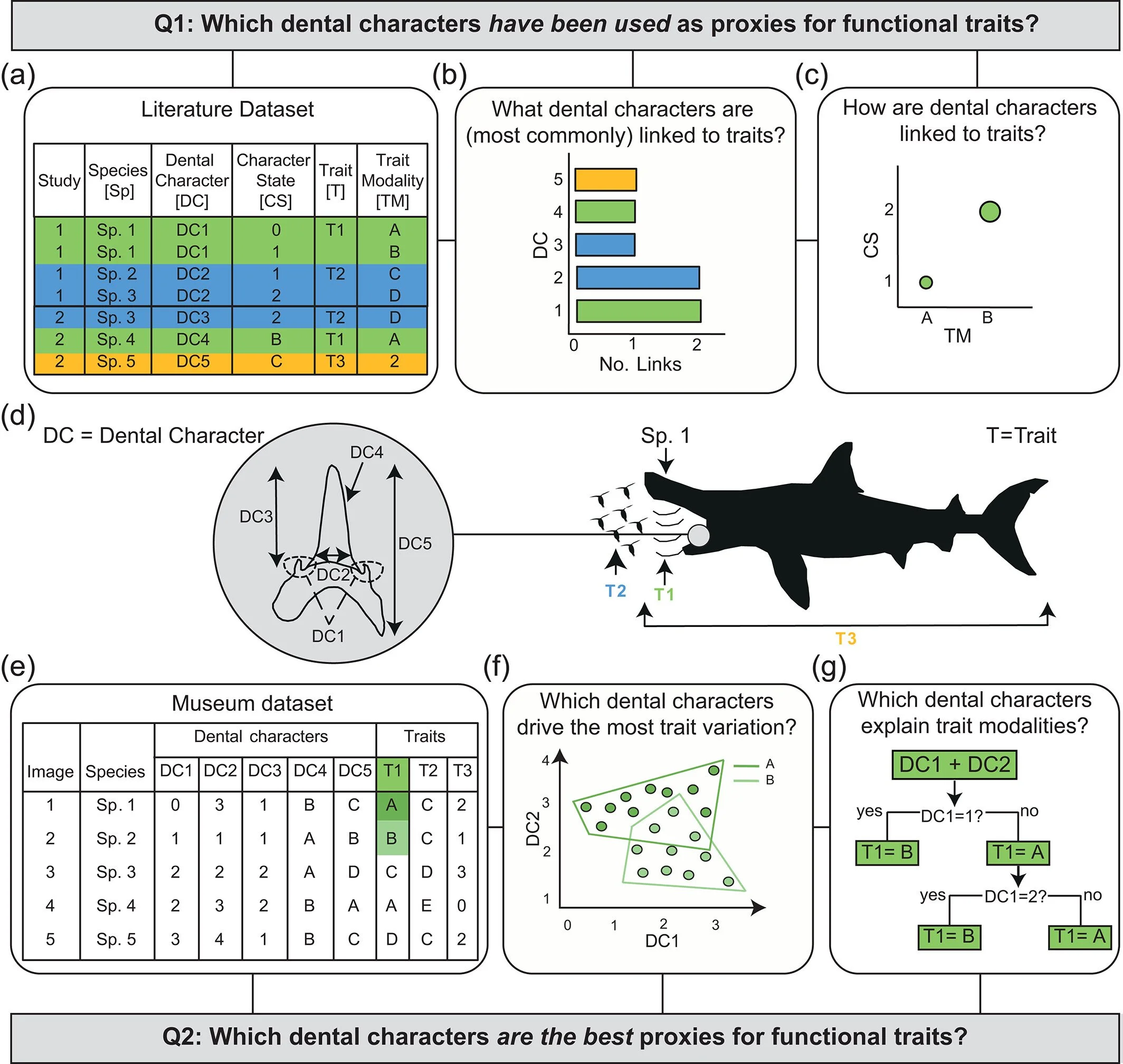
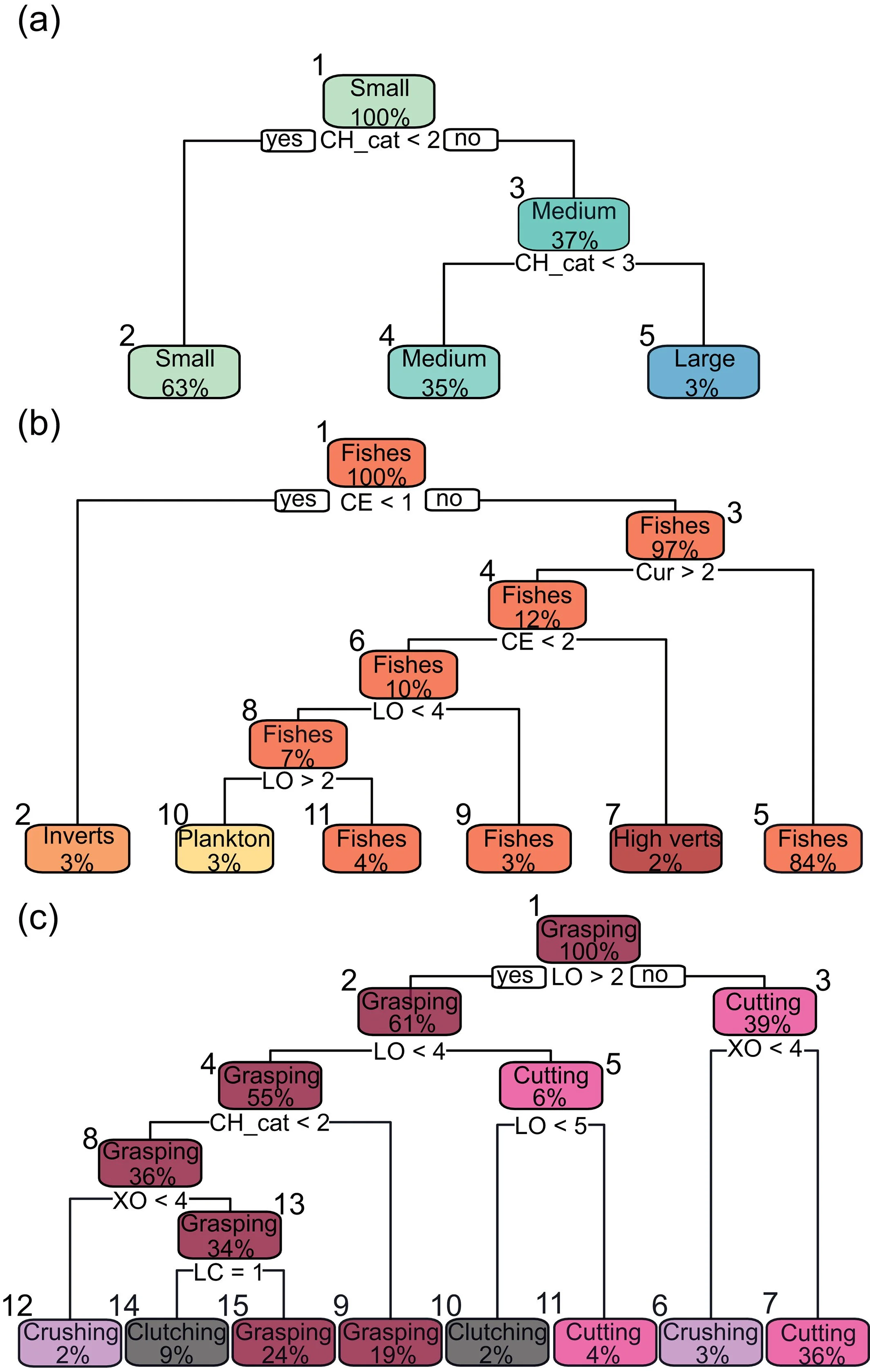
To do this, I first conducted a literature review identifying papers that had previously used proxies for three traits: (1) body size; (2) prey preference; and (3) feeding mechanism, investigating how often certain tooth measurements (or “dental characters” as we called it in the paper) were linked to these traits, and exactly how they were linked to each other. Following this, I conducted two validation analyses to find out which dental characters identified in the literature review were the best proxies for the three traits. Namely, I used a principal component analysis (PCA) to see which dental characters drove variation in traits, and a classification tree analysis to see which tooth measurements explained certain trait values best (Figure 1). There were a lot of dental characters looked at across multiple living species, and there’s a whole paper you can read that goes into the full details (Cooper et al. 2023). However, to summarise the key takeaway, tooth size was generally a good proxy for all three traits, particularly body size, the presence of serrated cutting edges or lateral cusplets were the best indicators of prey preference, and tooth shape was an imperfect but good predictor of feeding mechanism (Figure 2; Cooper et al. 2023). The work ultimately produced a framework to infer ecology from dental characters in isolated teeth.
Figure 1. The conceptual approach of my first PhD chapter (Cooper et al. 2023). Q1 is answered using the following steps: (a) taxonomic, dental character (DC) and corresponding functional trait (T) data are extracted from the literature; (b) the dental characters most commonly and broadly used as proxies for individual traits are identified; and (c) individual links between dental character states (CS) and trait modalities (TM) are quantified. (d) A graphical example of dental characters and their use as proxies for functional traits. Q2 is answered using the following steps: (e) dental characters and trait values are recorded from jaw specimens from museum collections; and validation analyses performed on this data, specifically (f) PCA to identify which dental characters drive trait variation; and (g) classification tree analysis to find which dental characters best explain trait values. This figure is sourced from Figure 1 of Cooper et al. (2023).
Figure 2. Classification tree analyses on dental characters recorded from the museum data set. Each tree is related to a single functional trait as follows: (a) body size, (b) prey preference and (c) feeding mechanism. Nodes are produced by splitting the data based on the presence of the dental character states recorded as predictors. The proportional node contributions to the entire data set are included alongside the most common trait value making up each node. This figure is sourced from Figure 6 of Cooper et al. (2023).
Following this chapter’s completion, and subsequent publication, I applied the framework to over 500 species from the Cenozoic era – ranging from fossils of up to 66 million years old to teeth from extant sharks. This allowed me to show precisely how shark functional diversity has changed over the last 66 million years. Specifically, I found that it was relatively high for 50 million years, before declining over the last 10 million years to its lowest value today; with extinct sharks generally having a larger functional diversity than living sharks (Figure 3). The reason we focused on the Cenozoic era was simple. Many fossil sharks from this time have living representatives (Paillard et al. 2020; Pimiento & Benton 2020), meaning that the links between dental characters and trait values can be reasonably applied without making any drastic assumptions about the extinct species.
Figure 3. Functional space of Cenozoic sharks as investigated by my second PhD paper. (a) Structure of the three-dimensional functional space for all sharks. Black dots represent the highest and lowest scoring taxon per axis, with their corresponding teeth illustrated and numbered following an accompanying legend. Grey dots mark all other taxa. (b–h) Shark functional spaces over time, with the volume of space occupied in each time bin (i.e., functional richness) depicted by coloured convex hulls. Coloured dots denote taxa present in each assemblage, while grey dots represent absent taxa. Turquoise and orange dots denote taxa with the highest FOri and FSpe scores, respectively, which are detailed in the legend. (i) Convex hulls of extinct (blue) and extant (grey) sharks. This figure is sourced from Figure 1 of Cooper & Pimiento (2024).
Applying the framework beyond its intended use
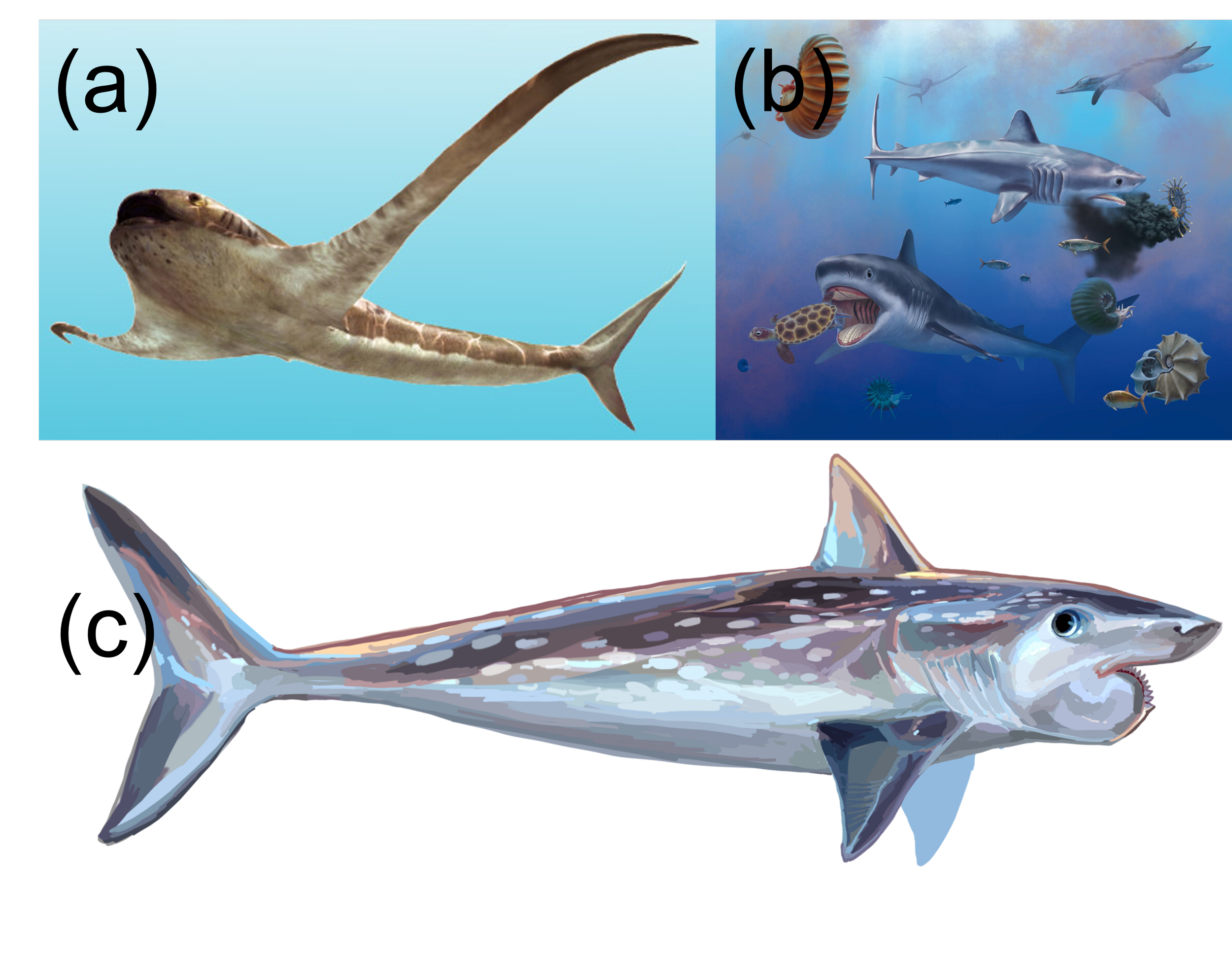
On paper, it made sense during my PhD not to go even further back in time to use sharks from the Mesozoic (252-66 million years ago) or even the Palaeozoic era (539-252 million years) for functional diversity analyses. It’s because there are several shark species known from more complete remains beyond isolated teeth that were nothing like any living shark (Figure 4). Take Aquilolamna milarcae for example: a Cretaceous shark with a torpedo-like body and wing-like pectoral fins that make it resemble a manta ray more than a shark (Vullo et al. 2021). Also from the Cretaceous is Ptychodus, a 10 m long giant that resembled lamnid sharks but had flat, pavement-like “crushing” teeth for likely eating shelled vertebrates like ammonites (Vullo et al. 2024). This is despite similar teeth seen in living sharks that feed on shelled invertebrates or even seagrass (Ciampaglio et al. 2005; Leigh et al. 2018). And in the Palaeozoic, even further back in time, there is of course Helicoprion with its strange whorl teeth not seen in any other shark (Tapanila et al. 2013). With all these bizarre or unique adaptations not seen in today’s sharks, it makes one wonder how the framework from my first PhD chapter (Cooper et al. 2023) could ever be used in the weird old sharks.
Yet, over the last few months, a pair of very intriguing studies have done just that.
Figure 4. Some bizarre Mesozoic and Palaeozoic sharks unlike any living shark. (a) Aquilolamna milarcae, reported by Vullo et al. (2021). Artwork by Oscar Sanisidro. (b) Ptychodus, reported by Vullo et al. (2024). Artwork by Frederik Spindler. (c) Helicoprion davisii. Artwork available on the Helicoprion Wikipedia page and credited to “Entelognathus”.
The Mesozoic study
One of these studies was published in April 2025 by Estevan Eltink and colleagues (Eltink et al. 2025). Specifically, it applied my earlier framework to a shark not only older than the living sharks I used, but also of a totally different order and family.
One of the most prominent kind of sharks in the Mesozoic was the hybodonts, of the order Hybodontiformes. Initially appearing in the Carboniferous period (during the Palaeozoic; ~360 million years ago), these sharks are widely known from Triassic and Jurassic deposits in the Mesozoic era (Kent 1994; Cappetta 2012). They’re notable for having fin spines and sometimes even horn-like structures on their heads (Figure 5), but they also just so happen to be the direct ancestors to modern sharks today – the neoselachians (Maisey 2012), a group that began to fully diversify from the Jurassic onwards (Guinot & Cavin 2016; Sternes et al. 2024; Gayford & Jambura 2025). Yet, seeing as we don’t have living representatives of these sharks, we have to use their fossils to deduce their ancient ecology.
“Teeth represent clear adaptations for feeding habits in sharks,” Estevan says, “the complex history of sharks can be told because of their teeth.”
As such, Estevan and his colleagues turned to the teeth, where my framework comes in.
Figure 5. Palaeoartistic reconstruction of male and female individuals of the hybodont Hybodus hauffianus. Artwork available on the Hybodus Wikipedia page and credited to “Gasmasque”.
Specifically, the authors looked at the species Priohybodus arambourgi from fossils in Brazil, a standout amongst the hybdonts for having both multiple cusplets and yet also being serrated on the cutting edges (Figure 6; Eltink et al. 2025). Serrated teeth tend to be present in larger living sharks feeding on large prey by cutting through flesh, yet they also do not tend to have lateral cusplets, typically used for grasping fish (Cooper et al. 2023). By analysing 153 teeth and running PCA on these teeth from Brazil, Uruguay and Libya, Estevan and his colleagues were able to find that, despite the unusual tooth morphology, the size and shape of P. arambourgi teeth still generally aligned with modern sharks. Indeed, lamniform sharks were found to be ecologically similar by occupying the same general morphospace as P. arambourgi. Moreover, wear found on the teeth very much indicated a cutting-like motion similar to what we would see in living sharks with serrated teeth (Whitenack & Motta 2010; Cooper et al. 2023). As such, the authors were able to apply my framework to P. arambourgi. In conclusion, they found that the tooth sizes indicated a “medium” size shark (i.e., around 2-4 m; Shimada et al. 2020; Cooper et al. 2023) and likely had a “cutting” feeding mechanism for most likely eating large fish. In short, by analysing the fossil teeth in comparison with other sharks with living representatives; the authors could use this to help apply my framework to this Mesozoic species.
Figure 6. Teeth of Priohybodus arambourgi from the Aliança Formation of Brazil (Upper Jurassic) with scale bars marking 5 mm. This figure is sourced from Figure 3 of Eltink et al. (2025).
Estevan summarises the main findings quite well by telling me, “It seems that Priohybodus shared a similar ecology with living shark predators. However, some questions are still open.”
The Palaeozoic study
The other study, focusing on the Late Devonian shark Ctenacanthus concinnus, was published in January 2025 (Greif et al. 2025) and led by Merle Greif, a PhD researcher at the University of Zurich where several of my peers in the Pimiento Research Group were based. Her study aimed to understand more about the feeding ecology of ctenacanths like C. concinnus, which are known from body fossils to have been large predators but yet remain understudied from an ecological standpoint. The paper opens by referring to the framework from my own PhD but then goes on to make a very compelling point: that morphology alone from teeth can only tell so much. A tooth’s shape might imply a certain feeding mechanism, but additional biomechanical analyses are key to truly unravelling precisely how an extinct shark might have fed. This is even more important when you consider that there are no living sharks like C. concinnus that could help deduce feeding ecology.
“I honestly think it is hard to tell whether teeth make good proxies or not for early chondrichthyans at this point,” says Merle, “There might be a lot of evolution in between [fossil sharks and living ecological analogues] and yet they are the best we have to make comparisons.”
And so, Merle and her colleagues set out to conduct those very analyses, focusing on teeth from Morocco.
Figure 7. The tooth of Ctenacanthus concinnus, sourced from Figure 1 of Greif et al. (2025); and a palaeoartistic reconstruction of the shark, found on the Ctenacanthus Wikipedia page and credited to “Gasmasque”.
Looking at nine teeth, Merle and her co-authors conducted dental microwear texture analysis – in which wear patterns are examined to assess diet. The same teeth were also subjected to finite element analysis. Here, the teeth were CT-scanned and loaded under different biting scenarios such as puncture, pulling and lateral shaking. These methods provided two crucial findings: (1) vertical scratches on the teeth suggested a combination of head shaking and a puncturing-style feeding, akin to the “grasping” mechanism described in my earlier paper; and (2) the highest stresses from FEA came under prey grasping, similar to modern sharks such as the snaggletooth shark (Hemipristis elongata). In short, the dental characters alone were not simply applied to the species here; but a combination of additional quantitative analyses allowed Merle and her co-authors to assess feeding mechanism much more directly; and supporting the tooth morphology’s “pointy” shape and lateral cusplets indicating a grasping feeding mechanism (Greif et al. 2025).
“All together we conclude that these early sharks have been opportunistic feeders, basically eating whatever swims in front of them,” Merle explains to me. On the importance of using additional methods beyond simple tooth measurements, she says: “Different methods gave us different hints to reconstruct diet and feeding and that’s why I think it is important to keep in mind that only looking at one thing can possibly lead to wrong or just incomplete assumptions.”
So what could my chapter’s framework be used for next?
When considering these two fascinating studies against my original work (Cooper et al. 2023); there’s one obvious thing they share that stands out to me. They didn’t just simply apply the framework from my paper; but went beyond that for their ecological inferences. By combining the framework with other analyses such as PCA, or FEA in the case of Merle’s paper, they were able to not only be more confident in their inferences; but have additional data to directly back them up. This is a great additional step to ensure we’re not overly relying on the word of a single paper or on a few tooth measurements, and I applaud these two papers for taking this initiative.
Although it’s tempting to feel vindication since these papers’ extra steps have mostly supported the tooth morphology links to traits from my paper (Cooper et al. 2023), it’s best to remain cautious when applying my framework to extinct sharks without living representatives. As such, I agree with these studies’ approaches to use take my framework a step further. Most importantly though, they remain within the wider point of my original framework – to infer ecology based entirely on data obtained from the fossil teeth themselves.
My second PhD chapter used teeth to assess how shark functional diversity changed across the entire Cenozoic era (Cooper & Pimiento 2024). I’m very proud of that paper and these new studies could one day allow somebody to expand on that work. It would be extremely ambitious, but with a combination of tooth morphology and additional analyses to verify tooth-trait links, perhaps somebody could eventually evaluate shark functional diversity across their entire 450-million-year evolutionary history. As Estevan puts it: “The shark history is vast, complex and beautiful!”
He adds: “I would argue the teeth may be an interesting starting-point for studies and ecological inferences, but concerning that biology of sharks does not resume to their teeth, further analyses and comparisons among extinct and living representatives would explore more in depth internal structures, or even macroevolutionary patterns, telling us this history in more detailed manner. So the science advances step by step.”
Merle further emphasises the importance of additional work. “I definitely think we can go further back than the Cenozoic but of course it is always better to include more information if possible. For Cenozoic sharks I think it is fair to use teeth as proxies. For older sharks and relatives, I think we have to be very careful in making clear assumptions,” she says, “I think it is just important to be open to changes and that our own work might not be valid anymore once new findings come up or new methods give a better picture.”
With how incomplete the fossil record is, and how large a scale such a task would be, I don’t see an extension of my earlier work to assess shark functional diversity into the Mesozoic and Palaeozoic happening any time soon. However, the data from my paper (Cooper & Pimiento 2024) are publicly available, as is the code used to conduct the functional diversity analyses. So, if anybody does decide to take on this task and wants to collaborate, hit me up!
Whatever the case, works like the two studies I’ve highlighted are important in showing how previous work can be incorporated into ecological analyses, but also how new steps are often the key to finding a more complete story from a fossil shark tooth.
References
Cappetta H, 2012. Handbook of Paleoichthyology—Chondrichthyes—Mesozoic and Cenozoic Elasmobranchii: Teeth. In H-P Schultze (Ed.), Handbook of paleoichthyology (Vol. 3E, pp. 1–512). Verlag Dr. Friedrich Pfiel.
Ciampaglio CN, Wray GA & Corliss BH, 2005. A toothy tale of evolution: convergence in tooth morphology among marine Mesozoic–Cenozoic sharks, reptiles, and mammals. The Sedimentary Record, 3, 4-8.
Cooper JA, 2024. Shark functional diversity through time: past, present and future. PhD thesis, Swansea University.
Cooper JA, Griffin JN, Kindlimann R & Pimiento C, 2023. Are shark teeth proxies for functional traits? A framework to infer ecology from the fossil record. Journal of Fish Biology, 103, 798-814.
Cooper JA & Pimiento C, 2024. The rise and fall of shark functional diversity over the last 66 million years. Global Ecology and Biogeography, 33, e13881.
Eltink E, da Silva KR, de França MAG, de Morais DMF, Soto M & Duffin CJ, 2025. Morphology and paleoecology of a hybodontiform with serrated teeth, Priohybodus arambourgi, from the Late Jurassic of northeastern Brazil. The Anatomical Record, 1-28.
Gayford JH, Engelman RK, Sternes PC, Itano WM, Bazzi M, Collareta A, Salas‐Gismondi R & Shimada K, 2024. Cautionary tales on the use of proxies to estimate body size and form of extinct animals. Ecology and Evolution, 14, e70218.
Gayford JH & Jambura PL, 2025. Drivers of diversification in sharks and rays (Chondrichthyes: Elasmobranchii). Frontiers in Ecology and Evolution, 12, 1530326.
Greif M, Calandra I, Lautenschlager S, Kaiser TM, Mezane M & Klug C, 2025. Reconstruction of feeding behaviour and diet in Devonian ctenacanth chondrichthyans using dental microwear texture and finite element analyses. Royal Society Open Science, 12, 240936.
Guinot G & Cavin L, 2016. ‘Fish’ (Actinopterygii and Elasmobranchii) diversification patterns through deep time. Biological Reviews, 91, 950-981.
Hubbell G, 1996. Using tooth structure to determine the evolutionary history of the white shark. In AP Klimley & DG Ainley (Eds.), Great white sharks: The biology of Carcharodon carcharias (pp. 9–18). San Diego: Academic Press.
Kent BW, 1994. Fossil sharks of the Chesapeake Bay region. Columbia, Maryland: Egan Rees & Boyer, Inc.
Leigh SC, Papastamatiou YP & German DP, 2018. Seagrass digestion by a notorious ‘carnivore’. Proceedings of the Royal Society B: Biological Sciences, 285, 20181583.
Maisey JG, 2012. What is an ‘elasmobranch’? The impact of palaeontology in understanding elasmobranch phylogeny and evolution. Journal of Fish Biology, 80, 918-951.
Mouillot D, Graham NA, Villéger S, Mason NW & Bellwood DR, 2013. A functional approach reveals community responses to disturbances. Trends in ecology & evolution, 28, 167-177.
Paillard A, Shimada K & Pimiento C, 2020. The fossil record of extant elasmobranchs. Journal of Fish Biology, 98, 445-455.
Pimiento C & Benton MJ, 2020. The impact of the Pull of the Recent on extant elasmobranchs. Palaeontology, 63, 369-374.
Shimada K, Becker MA & Griffiths ML, 2020. Body, jaw, and dentition lengths of macrophagous lamniform sharks, and body size evolution in Lamniformes with special reference to ‘off-the-scale’ gigantism of the megatooth shark, Otodus megalodon. Historical Biology, 33, 2543-2559.
Sternes PC, Schmitz L & Higham TE, 2024. The rise of pelagic sharks and adaptive evolution of pectoral fin morphology during the Cretaceous. Current Biology, 34, 2764-2772.
Tapanila L, Pruitt J, Pradel A, Wilga CD, Ramsay JB, Schlader R & Didier DA, 2013. Jaws for a spiral-tooth whorl: CT images reveal novel adaptation and phylogeny in fossil Helicoprion. Biology Letters, 9, 20130057.
Vullo R, Frey E, Ifrim C, Gonzalez Gonzalez MA, Stinnesbeck ES & Stinnesbeck W, 2021. Manta-like planktivorous sharks in Late Cretaceous oceans. Science, 371, 1253-1256.
Vullo R, Villalobos-Segura E, Amadori M, Kriwet J, Frey E, González González MA, Padilla Gutiérrez JM, Ifrim C, Stinnesbeck ES & Stinnesbeck W, 2024. Exceptionally preserved shark fossils from Mexico elucidate the long-standing enigma of the Cretaceous elasmobranch Ptychodus. Proceedings of the Royal Society B, 291, 20240262.
Whitenack LB & Motta PJ, 2010. Performance of shark teeth during puncture and draw: implications for the mechanics of cutting. Biological Journal of the Linnean Society, 100, 271-286.